Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 -hexaoctyl nitrilotriacetamide (HONTA) using mixer-settler extractors in a hot cell
-hexaoctyl nitrilotriacetamide (HONTA) using mixer-settler extractors in a hot cellBan, Yasutoshi; Suzuki, Hideya*; Hotoku, Shinobu; Tsubata, Yasuhiro
Solvent Extraction Research and Development, Japan, 31(1), p.1 - 11, 2024/00
A demonstration test was performed to separate minor actinides (MA; Am and Cm) by  -hexaoctyl nitrilotriacetamide (HONTA) as an extractant using mixer-settler extractors installed in a hot cell. A high-level liquid waste containing MA, and rare earths (RE; Y, La, Nd, and Eu) was used as the feed. HONTA diluted to 0.05 mol/dm
-hexaoctyl nitrilotriacetamide (HONTA) as an extractant using mixer-settler extractors installed in a hot cell. A high-level liquid waste containing MA, and rare earths (RE; Y, La, Nd, and Eu) was used as the feed. HONTA diluted to 0.05 mol/dm in
in  -dodecane was fed as the organic phase, and a part of the organic phase was reused without solvent regeneration. HONTA effectively extracted MA, whereas RE were less extractable. Consequently, the Y, La, Nd, and Eu ratios distributed to a RE fraction were
-dodecane was fed as the organic phase, and a part of the organic phase was reused without solvent regeneration. HONTA effectively extracted MA, whereas RE were less extractable. Consequently, the Y, La, Nd, and Eu ratios distributed to a RE fraction were  99.9%, 99.2%, 61.8%, and 81.4%, respectively. The Am and Cm ratios distributed to an MA fraction were 86.8% and 74.7%, respectively, and a substantial amount of MA (0.12 g) was recovered in the MA fraction by the end of the cumulative duration of 40 h.
99.9%, 99.2%, 61.8%, and 81.4%, respectively. The Am and Cm ratios distributed to an MA fraction were 86.8% and 74.7%, respectively, and a substantial amount of MA (0.12 g) was recovered in the MA fraction by the end of the cumulative duration of 40 h.
Matsuda, Shohei; Yokoyama, Keiichi
Isotope News, (786), p.6 - 9, 2023/04
no abstracts in English
Hirata, Sakiko*; Kusaka, Ryoji; Meiji, Shogo*; Tamekuni, Seita*; Okudera, Kosuke*; Hamada, Shoken*; Sakamoto, Chihiro*; Honda, Takumi*; Matsushita, Kosuke*; Muramatsu, Satoru*; et al.
Inorganic Chemistry, 62(1), p.474 - 486, 2023/01
Times Cited Count:0 Percentile:0.01(Chemistry, Inorganic & Nuclear)Ikusawa, Yoshihisa; Hirooka, Shun; Uno, Masayoshi*
2018 GIF Symposium Proceedings (Internet), p.321 - 327, 2020/05
Research and development of Minor actinides (MAs) bearing MOX fuel for fast reactor has been proceeding from the viewpoint of reducing radioactive waste. In order to develop, MA bearing MOX, it is indispensable to clarify the influence of MA addition on irradiation behavior. The addition of Americium (Am) to MOX affects vapor pressure and thermal conductivity, which are important properties from the perspective of evaluating fuel temperature. This is because vapor pressure affects fuel restructuring, and thermal conductivity affects fuel temperature distribution. Focusing on these physical properties, this study evaluates the influence of Am on fuel temperature using irradiation behavior analysis code to contribute to the development of MA-bearing MOX fuel. An increase in Am content decreases the thermal conductivity and increases the oxygen potential of oxide fuel. Because vapor pressure increases with increasing Am content, pore migration is accelerated, and the central void diameter increases with increasing Am content. As a result, after formation of the central void, the influence of Am content on the fuel center temperature is mild. Alpha particles generated by radioactive decay of transuranium elements cause lattice defects in the oxide fuel pellets. It is well known that this phenomenon, which is called self-irradiation, affects thermal conductivity. Since americium is the typical alpha radioactive nucleus, to evaluate fuel temperature of Am-MOX is necessary to take account of the influence of self-irradiation damage on thermal conductivity. Self-irradiation decreases thermal conductivity, and as the Am content increases, the rate of decrease in thermal conductivity is accelerated. Because it recovers with temperature rise, the decrease in thermal conductivity due to self-irradiation damage has very little effect on fuel center temperature. These results suggest that Am-MOX fuel could be irradiated under the same conditions as conventional MOX fuel.
Tsutsui, Nao; Ban, Yasutoshi; Suzuki, Hideya*; Nakase, Masahiko*; Ito, Sayumi*; Inaba, Yusuke*; Matsumura, Tatsuro; Takeshita, Kenji*
Analytical Sciences, 36(2), p.241 - 246, 2020/02
Times Cited Count:21 Percentile:79.8(Chemistry, Analytical)To investigate the effective separation of actinides (Ans) from lanthanides (Lns), single-stage batch extraction experiments were performed with a novel extractant, tetradodecyl-1,10-phenanthroline-2,9-diamide (TDdPTDA) with various diluents such as 3-nitrobenzotrifluoride (F-3), nitrobenzene, and  -dodecane for Am, Cm, and Lns. The extraction kinetics with TDdPTDA was rapid enough to perform the actual extraction flow sheet. The slopes of the distribution ratio versus TDdPTDA concentration and the distribution ratio versus nitric acid concentration were similar for F-3 and nitrobenzene systems but different from
-dodecane for Am, Cm, and Lns. The extraction kinetics with TDdPTDA was rapid enough to perform the actual extraction flow sheet. The slopes of the distribution ratio versus TDdPTDA concentration and the distribution ratio versus nitric acid concentration were similar for F-3 and nitrobenzene systems but different from  -dodecane system. These differences were attributed to the characteristics of the diluents. This study reveals high distribution ratios of Am (
-dodecane system. These differences were attributed to the characteristics of the diluents. This study reveals high distribution ratios of Am (
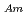 ) and Cm (
) and Cm (
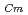 ) for TDdPTDA, with the high separation factors (
) for TDdPTDA, with the high separation factors ( s) of Am from Lns enough for their separation.
s) of Am from Lns enough for their separation.
 -hexaoctyl nitrilotriacetamide (HONTA) using mixer-settler extractors in a hot cell
-hexaoctyl nitrilotriacetamide (HONTA) using mixer-settler extractors in a hot cellBan, Yasutoshi; Suzuki, Hideya*; Hotoku, Shinobu; Tsutsui, Nao; Tsubata, Yasuhiro; Matsumura, Tatsuro
Solvent Extraction and Ion Exchange, 37(7), p.489 - 499, 2019/11
Times Cited Count:15 Percentile:52.81(Chemistry, Multidisciplinary)A continuous counter-current experiment to separate minor actinides (MAs: Am and Cm) was performed with  -hexaochyl nitrilotriacetamide (HONTA) as an extractant. Nitric acid of 0.08 M (mol/dm
-hexaochyl nitrilotriacetamide (HONTA) as an extractant. Nitric acid of 0.08 M (mol/dm ) containing MAs and rare earths (REs) recovered from high-level waste was used as the Feed, and the experiment was conducted for 14 h. The ratios of Am and Cm recovered into the MA fraction measured 94.9% and 78.9%, respectively. HONTA hardly extracted Y, La, and Eu in the Feed (99.9% for Y, 99.9% for La, and 96.7% for Eu), most of which were distributed to the RE fraction. A portion of Nd was extracted by HONTA, and consequently the ratio of Nd in the RE fraction was 83.5%. The concentrations of MAs and some REs in each stage were calculated using a simulation code, and the results are consistent with the experimental values. This code indicates that the ratios of MAs in the MA fraction and REs in the RE fraction could be
) containing MAs and rare earths (REs) recovered from high-level waste was used as the Feed, and the experiment was conducted for 14 h. The ratios of Am and Cm recovered into the MA fraction measured 94.9% and 78.9%, respectively. HONTA hardly extracted Y, La, and Eu in the Feed (99.9% for Y, 99.9% for La, and 96.7% for Eu), most of which were distributed to the RE fraction. A portion of Nd was extracted by HONTA, and consequently the ratio of Nd in the RE fraction was 83.5%. The concentrations of MAs and some REs in each stage were calculated using a simulation code, and the results are consistent with the experimental values. This code indicates that the ratios of MAs in the MA fraction and REs in the RE fraction could be  99% by optimizing separation conditions.
99% by optimizing separation conditions.
Nakamura, Shoji; Terada, Kazushi*; Kimura, Atsushi; Nakao, Taro*; Iwamoto, Osamu; Harada, Hideo; Uehara, Akihiro*; Takamiya, Koichi*; Fujii, Toshiyuki*
Journal of Nuclear Science and Technology, 56(1), p.123 - 129, 2019/01
Times Cited Count:1 Percentile:11.15(Nuclear Science & Technology)Accurate data of  -ray emission probabilities are frequently needed when one quantitatively determines the amount of isotope by
-ray emission probabilities are frequently needed when one quantitatively determines the amount of isotope by  -ray measurements or obtains neutron capture cross-sections using them. Americium-243, one of the most important minor actinides, produces
-ray measurements or obtains neutron capture cross-sections using them. Americium-243, one of the most important minor actinides, produces  Am after neutron capture. The 744-keV
Am after neutron capture. The 744-keV  -ray decaying from the ground state of
-ray decaying from the ground state of  Am has a relatively large
Am has a relatively large  -ray emission probability c.a. 66%, however, its uncertainty is as large as 29%. The uncertainty of the
-ray emission probability c.a. 66%, however, its uncertainty is as large as 29%. The uncertainty of the  -ray emission probability leads to a major factor of the systematic uncertainty on determining an amount of isotope, and therefore the
-ray emission probability leads to a major factor of the systematic uncertainty on determining an amount of isotope, and therefore the  -ray emission probability was measured by using an activation method and an examined level structure of
-ray emission probability was measured by using an activation method and an examined level structure of  Cm. In this study, the emission probability of 744-keV
Cm. In this study, the emission probability of 744-keV  ray was derived as 66.5
ray was derived as 66.5 1.1%, and its uncertainty was improved from 29% to 2%.
1.1%, and its uncertainty was improved from 29% to 2%.
 -tetradodecyldiglycolamide (TDdDGA) by using mixer-settler extractors in a hot cell
-tetradodecyldiglycolamide (TDdDGA) by using mixer-settler extractors in a hot cellBan, Yasutoshi; Suzuki, Hideya; Hotoku, Shinobu; Kawasaki, Tomohiro*; Sagawa, Hiroshi*; Tsutsui, Nao; Matsumura, Tatsuro
Solvent Extraction and Ion Exchange, 37(1), p.27 - 37, 2019/00
Times Cited Count:21 Percentile:65.06(Chemistry, Multidisciplinary)A continuous counter-current experiment using TDdDGA was performed using mixer-settler extractors installed in a hot cell. Nitric acid containing minor actinides (MAs: Am and Cm), rare earths (REs: Y, La, Nd, and Eu), and other fission products (Sr, Cs, Zr, Mo, Ru, Rh, and Pd) was fed to the extractor. TDdDGA effectively extracted MAs and REs from the feed, while other fission products were barely extracted. The extracted MAs and REs were back-extracted by bringing them in contact with 0.02 mol/dm nitric acid, and they were collected as the MA-RE fraction. The proportions of MA and RE in the MA-RE fraction were
nitric acid, and they were collected as the MA-RE fraction. The proportions of MA and RE in the MA-RE fraction were  98% and
98% and  86%, respectively. These results demonstrated the applicability of TDdDGA as an extractant for MAs and REs.
86%, respectively. These results demonstrated the applicability of TDdDGA as an extractant for MAs and REs.
Miyamoto, Yutaka; Yasuda, Kenichiro
Journal of Nuclear and Radiochemical Sciences (Internet), 18, p.13 - 15, 2018/07
A sequential separation technique using an anion-exchange column developed in the previous works have the potential to completely separate picograms of Am from the lanthanides using mixtures of acetic acid, hydrochloric acid, and nitric acid as the eluents, without any functional ligands or special columns. This experimental result implies that ultra-trace actinides, including Am, Pu, U, and Th in environmental samples can be sequentially separated by combination of these mixed-media eluents and an anion exchange column.
Kihara, Yoshiyuki; Tanaka, Kosuke; Koyama, Shinichi; Yoshimochi, Hiroshi; Seki, Takayuki; Katsuyama, Kozo
NEA/NSC/R(2017)3, p.341 - 350, 2017/11
In order to investigate the effect of the addition of americium to MOX fuels on the irradiation behaviour, the "Am-1" program is being conducted at the JAEA. The Am-1 program consists of two short-term irradiation tests of 10-min and 24-h irradiation periods, and a steady-state irradiation test. The short-term irradiation tests and their post irradiation examinations (PIEs) have been successfully completed. To date, the data for PIE of the Am-MOX fuels focused on the microstructural evolution and redistribution behaviour of Am at the initial stage of irradiation have been obtained and reported. In this paper, the results obtained from the Am-1 program are reviewed and detailed descriptions of the fabrication and inspection techniques for the Am-MOX fuels prepared for the program are provided. PIE data for the Am-MOX fuels at the initial stage of irradiation have been accumulated. In this paper, unpublished PIE data for the Am-MOX fuels are also presented.
 revealed by
revealed by  O-NMR
O-NMRTokunaga, Yo; Nishi, Tsuyoshi; Nakada, Masami; Ito, Akinori*; Sakai, Hironori; Kambe, Shinsaku; Homma, Yoshiya*; Honda, Fuminori*; Aoki, Dai*; Walstedt, R. E.*
Physical Review B, 89(21), p.214416_1 - 214416_8, 2014/06
Times Cited Count:7 Percentile:31.93(Materials Science, Multidisciplinary)The magnetic phase transition near 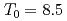 K in AmO
K in AmO has been investigated microscopically by means of
has been investigated microscopically by means of  O NMR. To avoid complexities arising from sample aging associated with the alpha decay of
O NMR. To avoid complexities arising from sample aging associated with the alpha decay of  Am, all measurements have been performed within 40 days after sample synthesis. Even during such a short period, however, a rapid change of NMR line shape has been observed at 1.5 K, suggesting that the ground state of AmO
Am, all measurements have been performed within 40 days after sample synthesis. Even during such a short period, however, a rapid change of NMR line shape has been observed at 1.5 K, suggesting that the ground state of AmO is very sensitive to disorder. We have also confirmed the loss of
is very sensitive to disorder. We have also confirmed the loss of  O NMR signal intensity over a wide temperature range below
O NMR signal intensity over a wide temperature range below 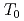 , and more than half of oxygen nuclei are undetectable at 1.5 K. This behavior reveals the persistence of slow and distributed spin fluctuations down to temperatures well below
, and more than half of oxygen nuclei are undetectable at 1.5 K. This behavior reveals the persistence of slow and distributed spin fluctuations down to temperatures well below 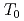 . In the paramagnetic state, strong NMR line broadening and spatially inhomogeneous spin fluctuations have been observed. The results are all indicative of short-range, spin-glass-like character for the magnetic transition in this system.
. In the paramagnetic state, strong NMR line broadening and spatially inhomogeneous spin fluctuations have been observed. The results are all indicative of short-range, spin-glass-like character for the magnetic transition in this system.
Hoshi, Harutaka*; Wei, Y.*; Kumagai, Mikio*; Asakura, Toshihide; Morita, Yasuji
Recent Advances in Actinide Science, p.596 - 598, 2006/06
Recently, extraction selectivity for trivalent minor actinides (MA = Am and Cm) over lanthanides (Ln) has been found in some extractants containing soft donor, such as S or N, ligands. Kolarik et al. reported that a new N-donor ligand 2,6-bis(5,6-dialkyl-1,2,4-triazine-3-yl)-pyridine (R-BTP) shows high selectivity for MA (III) over Ln(III) [1]. However, protonation of R-BTP results in its acidic hydrolysis in acidic medium. Stability in acidic solution was improved by substitution of long normal chain or branched chain [2]. In this work, separation of MA(III) and Ln(III) from nitric acid solution was studied by using novel R-BTP impregnated resin. Branched R-BTP resin had high affinity for Am from up to 4 M HNO solution and its distribution coefficient was over 10
solution and its distribution coefficient was over 10 .
.
Ueda, Masato; Sakamoto, Yoshiaki*
Genshiryoku Bakkuendo Kenkyu, 12(1-2), p.31 - 39, 2006/03
no abstracts in English
Hoshi, Harutaka*; Wei, Y.*; Kumagai, Mikio*; Asakura, Toshihide; Morita, Yasuji
Journal of Alloys and Compounds, 408-412, p.1274 - 1277, 2006/02
Times Cited Count:39 Percentile:84.47(Chemistry, Physical)For the development of advanced aqueous reprocessing system, it is one of the most important subjects to separate minor trivalent actinides (MA = Am and Cm). Recently, extraction selectivity for MA(III) over Ln(III) has been found in some extractants containing soft donor, such as S or N, ligands. Kolarik et al. reported that a new N-donor ligand 2,6-bis(5,6-dialkyl-1,2,4-triazine-3-yl)-pyridine (R-BTP) shows high selectivity for MA (III) over Ln(III). The novel silica-based extraction resins were prepared by impregnating some R-BTP molecules into a macroreticular styrene-divinylbenzene copolymer which is immobilized in porous silica particles with a mean diameter of 50  m. Separation of simulated high level liquid waste solution containing Ln(III) and trace amount of Am(III) was studied. Am(III) was mutually separated from Ln(III) through a packed column with R-BTP impregnating resin, very high decontamination factor (
m. Separation of simulated high level liquid waste solution containing Ln(III) and trace amount of Am(III) was studied. Am(III) was mutually separated from Ln(III) through a packed column with R-BTP impregnating resin, very high decontamination factor ( 10
10 ) for Am, and all the elements were recovered quantitatively.
) for Am, and all the elements were recovered quantitatively.
Ishimori, Kenichiro*; Watanabe, Masayuki; Kimura, Takaumi; Yaita, Tsuyoshi; Yamada, Takashi*; Kataoka, Yumiko*; Shinoda, Satoshi*; Tsukube, Hiroshi*
Chemistry Letters, 34(8), p.1112 - 1113, 2005/08
Times Cited Count:13 Percentile:44.77(Chemistry, Multidisciplinary)Stereochemical substitution on tripod ligand significantly offered efficient separation of trivalent actinides from trivalent lanthanides. Liquid-liquid extraction using chiral tris(2-pyridylmethyl)amine ligands as an extractant exhibited high efficiency and selectivity for trivalent actinides. A combination of chiral ligand and 2-bromodecanoic acid further enhanced extraction performance for trivalent actinides.
Arai, Yasuo; Pillon, S.*
Proceedings of International Conference ATALANTE 2004 Advances for Future Nuclear Fuel Cycles (CD-ROM), 9 Pages, 2004/06
no abstracts in English
 -decays of neutron-deficient americium isotopes
-decays of neutron-deficient americium isotopesSakama, Minoru*; Asai, Masato; Tsukada, Kazuaki; Ichikawa, Shinichi; Nishinaka, Ichiro; Nagame, Yuichiro; Haba, Hiromitsu*; Goto, Shinichi*; Shibata, Michihiro*; Kawade, Kiyoshi*; et al.
Physical Review C, 69(1), p.014308_1 - 014308_11, 2004/01
Times Cited Count:21 Percentile:73.68(Physics, Nuclear)no abstracts in English
Tanaka, Tadao; Sakamoto, Yoshiaki; Sawada, Hiroshi; Ogawa, Hiromichi
JAERI-Conf 2003-010, p.134 - 141, 2003/09
We have performed migration experiments of Np(V) and Am(III) for crushed granite, under the coexistent condition with humic acid substance. As for Np, the periodical concentration changes in the breakthrough curve and the migration velocity of Np passed through the column were not affected by the coexistence of the humic substance. As for Am, on the other hand, the periodical concentration changes in the breakthrough curve were affected by the humic substance concentration. The migration behavior of Am passed through the present column system could be expressed by a migration model taking account of the non-equilibrium state.
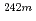 Am,
Am,  Am,
Am,  Tc and
Tc and 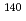 Ce
CeNakagawa, Tsuneo; Iwamoto, Osamu; Hasegawa, Akira
JAERI-Research 2002-035, 94 Pages, 2002/12
no abstracts in English
Kato, Hiroshige*; Mine, Tatsuya*; Mihara, Morihiro; Oi, Takao; Honda, Akira
JNC TN8400 2001-029, 63 Pages, 2002/01
Cementitious materials will be used for the TRU waste repository as a component of engineered barrier system. The distribution coefficients which represent the retardation of radionuclides migration for the cementitious materials would be one of the important parameter for the safety assessment. The much information of radionuclide sorption onto the cementitious materials has been accumulated through the study in the world. Therefore it is necessary to compile the information and Kd of the radionuclides reported in previous studies. In this report, the Kd of the important radionuclides, such as C, Ni, Se, Sr, Zr, Nb, Mo, Tc, Sn, I, Cs, Sm, Pb, Ra, Ac, Th, Pa, U, Np, Pu, Am, Cm, for the cementitious materials were compiled as the Sorption Database (SDB). For radionuclides to be sensitive to the redox potential, e.g. Se, Tc, Pa, U, Pu and Np, some Kds measured under the controlled atmosphere had been reported, and few Kds measured under the controlled redox potential had been reported. For Se, Mo, Sm, Cm and Ac, the distribution coefficients had not been reported, therefore distribution coefficients of Se and Mo for OPC (Ordinary Portland Cement) pastes were measured by batch sorption experiments and these data were added into the SDB.